
Back التطعيم ضد فيروس كورونا في كندا Arabic Vacunación contra la COVID-19 en Canadá Spanish Вакцинація проти COVID-19 у Канаді Ukrainian
This article's lead section may be too long. (December 2022) |
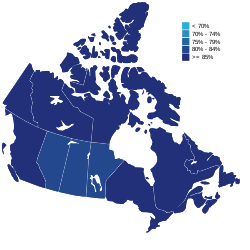 Percentage of the population vaccinated with at least one dose as of September 30, 2022 | |
| Date | December 14, 2020 – present |
|---|---|
| Location | Canada |
| Also known as | Vaccins contre la COVID-19 (French) |
| Cause | COVID-19 pandemic in Canada |
| Organized by | Health Canada Public Health Agency of Canada Provincial and Territorial governments Municipal government in Canada |
| Participants | 32,663,177 people with at least one dose administered of Pfizer, Moderna, AstraZeneca, Johnson & Johnson or Novavax 31,392,262 fully vaccinated people (to which the first and second doses of vaccine were administered) 19,250,351 people with a booster dose [1] |
| Outcome | 85.41% of the Canadian population has received at least one dose of a vaccine [1] 82.08% of the Canadian population is fully vaccinated 49.80% of the Canadian population has received at least one booster dose |
| Website | Government of Canada |
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
COVID-19 vaccination in Canada is an ongoing, intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic in Canada. Provinces have worked with local municipal governments, hospital systems, family doctors and independently owned pharmacies to aid in part, or in full with vaccination rollout.[2] The vaccination effort in full is the largest such immunization effort in the nation's history. The vaccination effort began December 14, 2020, and is currently ongoing.[3]
Health Canada is responsible for approval and regulation of vaccines (and other pharmaceuticals), while the Public Health Agency of Canada (PHAC) is responsible for public health, emergency preparedness and response, and infectious and chronic disease control and prevention. Vaccines are authorized by Health Canada, purchased by the Government of Canada and distributed by PHAC to individual provinces and territories in tranches based on various factors such as population size and prioritized peoples. The National Advisory Committee on Immunization (NACI) has also issued recommendations on how vaccines should be distributed, in what intervals and to which populations. NACI has also been involved in recommendations on the use or disuse of vaccines to certain ages or populations.
The National Research Council Canada (NRC) has made investments in the domestic development of vaccine candidates, including candidates by the University of Saskatchewan and Variation Biotechnologies. In May 2020, the NRC announced a planned agreement to conduct clinical trials of a vaccine candidate by Chinese company CanSino Biologics, and plans to manufacture it at its facilities in Montreal once authorized. However, the deal collapsed due to strained Canada–China relations, and the federal government later announced commitments to purchase vaccines being produced by AstraZeneca, Moderna, Pfizer and Janssen.
In early 2021, both Pfizer–BioNTech and Moderna did not ship the agreed upon quantities of secured vaccines to Canada and other countries, due to manufacturing challenges.[4] This caused a vaccine shortage and significant slowdown in vaccine rollout. By mid-February 2021, significant increases in manufacturing and delivery of vaccines in conjunction with a recommendation by NACI to extend second dose administration to a maximum of 16 weeks resulted in a larger ramp-up in vaccine delivery across the nation and by July 2021, Canada's vaccine supply had grown to allow a return to shortened dose intervals.[5]
Following Health Canada's emergency authorization of the Pfizer–BioNTech vaccine on December 9, 2020, mass vaccination efforts began across the country on December 14, 2020. The agency later authorized the Moderna vaccine on December 23, 2020. On February 26, 2021, Health Canada authorized the Oxford–AstraZeneca vaccine for use. Following concerns of blood clotting events the Oxford-AstraZeneca vaccine was largely discontinued for use, and those who had already received a first dose were encouraged to receive an mRNA vaccine as their second dose.[6] The Janssen vaccine was authorized on March 5, 2021; however, Canada did not receive a delivery of the Janssen vaccine until April 28, 2021;[7] which was then destroyed due to contamination issues at its factory of origin.[8][9] Use of Janssen was put on hold until November 2021, when the government acquired doses for use with vaccine-hesitant populations.[10]
Canada became the first country to authorize a COVID-19 vaccine for people younger than 16 after approving Pfizer's vaccine for children aged 12 to 15 on May 5, 2021.[11] In August 2021, the Moderna vaccine was authorized for use in children aged 12 and up.[12] On September 16, 2021, Health Canada granted full approval to the Moderna and Pfizer vaccines and in November 2021, Health Canada approved the Pfizer-BioNTech and Moderna vaccines for use as booster (or third doses).[13][14] On November 19, 2021, Health Canada approved the Pfizer-BioNTech vaccine (with a lower dosage) for children aged five to eleven.[15] On February 17, 2022, Health Canada approved the Novavax vaccine, which is the first approved COVID-19 protein subunit vaccine.[16]
- ^ a b "COVID-19 vaccination coverage in Canada". January 15, 2021. Retrieved October 5, 2022.
- ^ "Which COVID-19 vaccine booking system is your public health unit using?". CP24. March 3, 2021. Archived from the original on March 22, 2021. Retrieved March 22, 2021.
- ^ "Most Canadians will get COVID-19 vaccine by September: Trudeau". Al Jazeera. November 27, 2020. Archived from the original on February 17, 2021. Retrieved March 22, 2021.
- ^ Cite error: The named reference
CBC_Tasker_20210204was invoked but never defined (see the help page). - ^ "Canada to reach 55M vaccine doses by week's end, catching up to U.S. on second doses". CTV News. The Canadian Press. July 12, 2021. Archived from the original on July 12, 2021. Retrieved July 12, 2021.
- ^ Miller, Adam (June 1, 2021). "Canada recommends mixing and matching AstraZeneca, Pfizer and Moderna COVID-19 vaccines". CBC News. Archived from the original on June 1, 2021. Retrieved August 24, 2021.
- ^ "COVID-19 news today: Military to provide medical personnel to help Ontario's health-care system". The Globe and Mail. The Canadian Press. April 26, 2021. Archived from the original on May 6, 2021. Retrieved April 30, 2021.
- ^ "Canada awaiting results of U.S. probe before deciding plans for J&J vaccines". CTV News. The Canadian Press. May 21, 2021. Archived from the original on May 30, 2021. Retrieved May 25, 2021.
- ^ Neustaeter, Brooklyn (June 11, 2021). "Health Canada not releasing more than 300K doses of J&J vaccine over possible quality control issue". CTV News. Archived from the original on July 1, 2021. Retrieved June 28, 2021.
- ^ Thurton, David (November 5, 2021). "Canada obtaining Johnson & Johnson doses as it works to vaccinate the holdouts". CBC News. Retrieved November 5, 2021.
- ^ Gillies, Rob (May 5, 2021). "Canada authorizes Pfizer vaccine for age 12 and older". The Washington Post. ISSN 0190-8286. Archived from the original on August 24, 2021. Retrieved May 5, 2021.
- ^ Freeman, Joshua (August 27, 2021). "Health Canada authorizes Moderna COVID-19 vaccine for use in youth 12-17". CP24. Retrieved August 27, 2021.
- ^ "Health Canada authorizes Moderna COVID-19 vaccine as a booster shot". November 12, 2021. Archived from the original on November 12, 2021. Retrieved November 23, 2021.
- ^ Young, Leslie (November 9, 2021). "Pfizer COVID-19 booster dose authorized by Health Canada for all adults 18+ - National". Global News. Retrieved November 19, 2021.
- ^ Young, Leslie (November 29, 2021). "Pfizer COVID-19 vaccine for children aged 5-11 approved by Health Canada". Global News.
- ^ "Novavax's COVID-19 vaccine approved for Canadians 18 and over". CBC News. February 17, 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search